Short Notes # 5 (Revised)
Silane Coupling Agents
By
Sin Siew Weng, FIM, FPRIM, AMIC, ANCRT, FIManf, F.Prof
BTM.
Sin Siew Mun, BSc, ANCRT, APRIM
Sin Yoong Cheong, MEng
Sin Yoong Leong, BSc
Lee Aik Kwong, PhD, FPRIM, FMIC, ANCRT
First Published: 1999
Revised : 31 May 2000
2nd Revision 9 November 2000
1.Introduction
Silanes have been around for over 40 years but their early applications are more in adhesives and coatings. In rubber compounding, the first silane coupling agent was commercialised in 1971, the well known Si-69 by Degussa. This was to rectify the short comings of precipitated silica, introduced only about 1951, mainly poorer processing/ dispersion, poorer abrasion resistance compared to carbon blacks and is still expensive to use and until 1992, found only niche applications such as in high end transparent coloured shoe solings. With the introduction of the ‘Green Tyre’ concept by Michelin in 1992, silane coupling agents became hot chemicals and we now have several tailor-made silane coupling agents to select from.
The chemistry of silane coupling agents, its practical applications and its selection for each formulation are still not easy to comprehend.
Silane coupling agents are bifunctional organosilanes which can be represented viz:-
X-R-Si (OR’)3-n
where
X = organo functional groups which can form strong covalent crosslink with polymers.
Examples:- Polysulphides
Disulphides
Amino
Mercapto
Vinyl
Epoxy
Methacryl
Others
n = 0,1 or 2
OR’ = hydrolysable group capable of forming strong covalent bonds with the hydroxyl groups on silica surfaces such as:-
CL
OCH3
OC2H5
OC2H4OCH3
Others
R = methylene group
Bifunctional organosilanes therefore permit chemical cross linking between polymer and filler such as silica, silicate, talc, mica, clay an whiting which contains surface hydroxyl groups – hence their appropriate name silane coupling agents.
The basic chemistry seems simple enough, but the selection of each silane coupling agent to suit the polymer and/ or its curing system, the effects of other additions that can compete with silanes on their reactivity with the silanol groups on the silica surfaces, the different rates of silanes reactions and their effect of curing characteristics etc make their application a bit more complicated.
2. Commercial Silane Coupling Agents
At least nine classes of silane coupling agents are known.
|
Class |
Commercial
Names |
Abbreviations |
|||
|
1. Polysulfide Class |
|
|
|||
|
Bis-[3-(triethoxysilyl) propyl ] - tetrasulfide |
Degussa Si-69 |
TESPT |
|||
|
UC Silane A1289 |
|||||
|
Witco A1289 |
|||||
|
Uniroyal/ Witco RC2 |
|||||
|
Behn Meyer Couplink 89 |
|||||
|
Hung Pai HP 669 |
|||||
|
SRT TESPT |
TESPT-50G |
||||
|
|
|
|
|||
|
Bis-[3-(triethoxysilyl) propyl ] – disulfide |
Degussa VP
Si-75 |
TESPD |
|||
|
Witco 1589 |
|||||
|
Hung Pai HP 1589 |
|||||
|
|
|
|
|||
|
2. Mercapto Class |
|
|
|||
|
3-mercaptopropyltrimethoxysilane |
UC A189 |
MPTMS |
|||
|
|
|
|
|||
|
3-mercaptopropyltriethoxysilane |
UC A1891 |
MPTES |
|||
|
Hung Pai HP 1891 |
|||||
|
|
|
|
|||
|
3. Amino Class |
|
|
|||
|
3-aminopropyltriethoxysilane |
UC A1100 |
APTES |
|||
|
Witco A1100 |
|||||
|
|
|
|
|||
|
N-2-(aminoethyl)-3-amino propyltrimethoxysilane |
UC A1120 |
AEAPTMS |
|||
|
Witco A1120 |
|||||
|
|
|
|
|||
|
4. Chloro Class |
|
|
|||
|
|
Degussa Si 230 |
|
|||
|
|
|
|
|||
|
5. Vinyl Class |
|
|
|||
|
Vinyltrimethoxysilane |
UC A171 |
|
|||
|
Witco A171 |
|
||||
|
|
|
|
|||
|
Vinyl-tris(2-methoxyethoxy) silane |
UC A172 |
|
|||
|
Degussa Si-225 |
|
||||
|
Witco A172 |
|
||||
|
6. Methacrylate Class |
|
|
|
|||
|
3-methacryloxypropyltrimethoxy silane |
UC A174 |
|
|
|||
|
Witco A174 |
|
|||||
|
|
|
|
|
|||
|
7. Epoxy Class |
|
|
|
|||
|
2-(3,4-epoxycyclohexy)-ethyl trimethoxysilane |
UC A186 |
|
|
|||
|
Witco A186 |
|
|
||||
|
|
|
|
|
|||
|
3-glycidoxy – propyltriethoxysilane |
UC A187 |
|
|
|||
|
Witco A187 |
|
|
||||
|
|
|
|
|
|||
|
8. Isocyanato Class |
|
|
|
|||
|
3-isocyanatopropyltriethoxysilane |
UC A1310 |
|
|
|||
|
Witco A1310 |
|
|
||||
|
|
|
|
|
|||
|
9. Thiocyanato Class |
|
|
|
|||
|
3-cyanatopropyltriethoxysilane |
Degussa Si 264 |
TCPTES |
|
|||
|
Hung Pai HP 264 |
|
|||||
3. General Guidelines On Selection Based On Reactivity Of X
|
Polymers |
Reactivity Of X |
Silanes Recommended |
|
1. Unsaturated Polymers. E.g. NR, BR, SBR, NBR, EPDM, etc |
Free Radical/ Ionic |
Polysulfides, Mercapto, Thiocynato, Amino. |
|
|
|
|
|
2. Peroxide Cured |
Free Radical |
Vinyl & Methacrylate |
|
|
|
|
|
3. Halogenated Polymers. E.g. CR, CPE, CSPE |
Ionic |
Chloro or Mercapto |
|
|
|
|
|
4. PU’s |
Condensation |
Epoxy,
Isocyanato |
|
|
|
|
|
5. Unsaturated Polymers |
Free Radical |
Vinyl, Methacrylate, Epoxy |
4. Chemistry
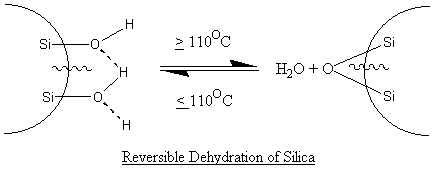 Silica, silicates, talc, mica, clay and whiting contain adsorbed
water and hydroxyl groups. The surface chemistry of silica has been well
studied and can be taken as representation. Silica surfaces contains siloxane,
isolated hydroxyls and hydrogen bonded hydroxyls. Silica contains <
0.5% moisture on manufacture and is known to be able to pick up as much as 6%
moisture before its final use. The hydration and dehydration of silica is
reversible viz:-
Silica, silicates, talc, mica, clay and whiting contain adsorbed
water and hydroxyl groups. The surface chemistry of silica has been well
studied and can be taken as representation. Silica surfaces contains siloxane,
isolated hydroxyls and hydrogen bonded hydroxyls. Silica contains <
0.5% moisture on manufacture and is known to be able to pick up as much as 6%
moisture before its final use. The hydration and dehydration of silica is
reversible viz:-
At > 110OC, the release of water creates more siloxane groups on the silica particle surface whilst at < 110OC, the free water reacts once more with the siloxane groups to form the silanols or hydroxyl groups or hydrogen-bonded-hydroxyls. These silanol groups behave like carboxylic acid and can react with example, amines, alcohol, metal salts etc.
The (-OR’)3 group ion silanes can thus react with the silica surfaces thus by first being hydrolysed with the hydroxyl groups present on the silica surfaces and then the chemical bonding reaction viz:
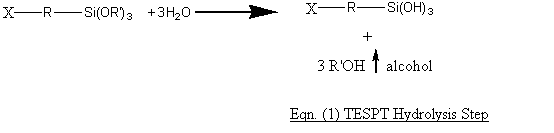
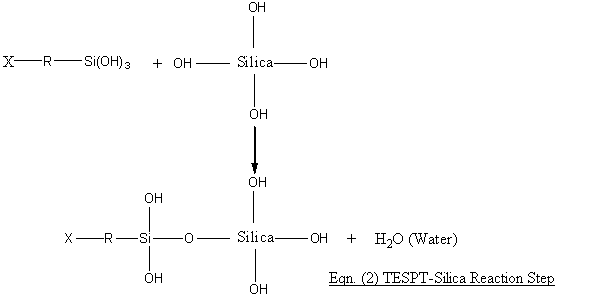
The choice of ethoxyl group in silane OR’ is obvious as the ethoxyl groups can react with the hydroxyls to liberate an alcohol which can readily be flashed off during mixing of 1st Step masterbatch. For Eqn. (1) to proceed, the mixing temperature should preferably be > 110OC to enable the adsorbed moisture on the silica surfaces to be liberated. This reaction is fast at > 110OC. The second Eqn. (2) reaction is relatively slow and since water is liberated, this reaction has to be completed and the liberated water flashed off. For TESPT, a second remill mixing step is often recommended whereby the masterbatch temperature is 160OC before it is dumped. Higher temperatures can cause TESPT to liberate some of its sulphur (TESPT is a sulphur donor as well) and cause scorch and premature vulcanisation.
The TESPD, MPTMS, MPTES, TCPTES and the amino class do not have above problem and 1st Step masterbatch can even be mixed up to 180OC to flash of the liberated water.
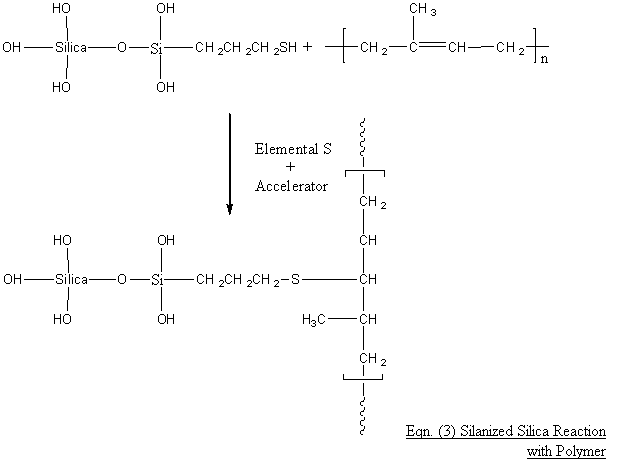
All the 5 sulphur containing silanes TESPD, TESPT, MPMTS, MPTES & TCPTES can then react during sulphur curing/ vulcanisation after Eqn. (2) with the polymer viz:
Example for MPTES:
For peroxide cured vinyl silane treated silica filled NR formulation, the reaction of the silane vinyl groups with NR is viz:-
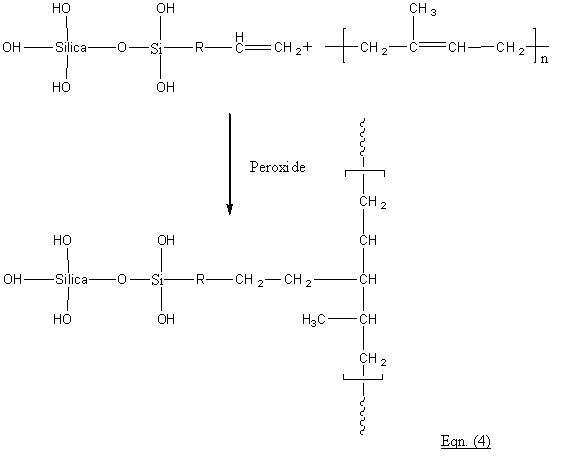
Readers are advised to consult our Short Notes #11 for further information on peroxide curing.
5. Theoretical Considerations In The Use OF Silane Coupling Agents.
Although the basic chemistry of silanes has been described, there are many theoretical and practical areas which are still not clear or pose problems. Take the more studied TESPT with sulphur cured unsaturated polymers in silica filled formulations. The functionalities of the (OR’) & X groups are accepted as illustrated in Equations 1 – 4. Questions can be asked.
1. It is not confirmed whether TESPT is Eqn. (1) reacts with the free water or more probable from water liberated by heat > 110OC and/ or mechanical shear from the silica hydroxyl groups.
2. It is idealistic that each molecule of TESPT can form a cross-link between one particle of silica and one polymer chain. Statistically, one particle of silica can react with X molecules of TESPT and 1 or more of these can cross-link with polymer chain or chains.
3. The (OR’)3 group on the TESPT has to compete with other formulation additives such as glycols, amines and accelerator containing amino, bivalent metallic soaps such as Zn or Mg stearates and other silanol hydroxyable functional compounds.
4. The very reinforcing nature of silica depends on its hydroxyl group which can form hydrogen bonding between silica particles. Removal of all these hydroxyl groups by treating silica with e.g. dimethyldichlorosilane or hexamethyldisilizane renders the silica hydrophobic and such silicas do not show reinforcing properties but behave more like inert fillers with poor abrasion resistance etc.
5. The ideal solution seems to be just enough TESPT to react with each silica particle surface but leaving other hydroxyl groups on its surface to form hydrogen bonding with an adjacent silica particle.
6. From above, it seems a fruitless exercise to attempt to solve the high viscosity or ‘crepe hardening’ problem even in silane treated silica formulations. One can however attempt to strike a compromise ie. after the silanization reaction, to allow sufficient additives like glycol or amines, to mop up sufficient remaining silanol groups on the silica to further reduce viscosity but yet maintaining the silica reinforcing characteristics.
7. That TESPT can split off free elemental sulphur at > 180OC and can cause scorch is well understood, but that TESPT does not accelerate cure as compared example with MPTMS or MPTES which can reduce t2 & t90 considerably, has not been fully elucidated.
8. Whether the reaction in Eqn. (1) can proceed at typical open mill temperatures < 110OC has not been proven satisfactorily yet.
9. Although some theoretical dosages of silane coupling agents needed per hundred parts silica of known particle size and surface activity are given, practical usage at varied increasing dosages do not give anticipated improvements e.g. in abrasion resistance or reduced viscosity. It is also not clear on molar basis, why the Mercapto class is more effective requiring lower dosages than e.g. TESPT. Correct practical dosages are best determined by trial and error.
10. The storage stability of almost all silanes are reckoned to be poor and recommendations are that once the air-tight seal is broken, the silane has to be used as soon as possible. Users are not given sufficient technical information on the rate of hydrolysis of silanes under ambient conditions and how different silanes compare.
11. Most silanes are insoluble in water at ambient conditions. Deliberate introduction of water into silane is like observing a drop of water into oil. The water globule floats and only turns opaque white and sinks after about 2 days, the rest of the silane remains unaffected & clear.
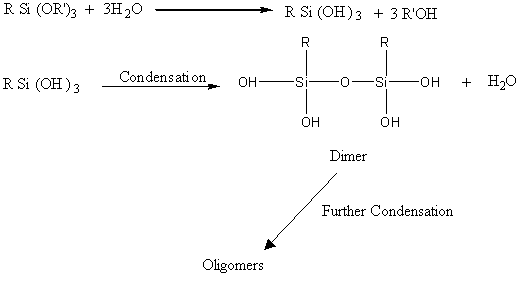 This slow hydrolysis of
silane is anticipated to go like this:-
This slow hydrolysis of
silane is anticipated to go like this:-
One can see that the initial hydrolysis of the silane is similar to Eqn. (1). Hence the initial stages of silane hydrolysis is not detrimental to its efficiency as a coupling agent, but once dimers are formed it is obviously less efficient but still effective and even less effective with further condensation of dimers to oligomers. Hence with visual observation of exposed silane, one can notice a gradual increase in viscosity from this liquid to a viscous liquid in about 3 months. It would appear that exposed silane under Malaysian ambient conditions (acidic moist air accelerates the hydrolysis) can be tolerated up to about 10 days. However for safety sake, use our polymer bound predispersed silanes which are guaranteed safe for at least 3 months exposure.
12. No doubt, the variable results obtained in silane treated silica formulations are due largely to the poor “dispersability” of standard proprietary silicas. Only recently we have the so-called highly dispersible (HD) silicas with better narrower control of particle size, specific surface area and the number of silanol groups as measured by moisture content.
13. It is recommended that silanes should be selected for its functional X group to match the cure system used. For example, in peroxide cure of say an NBR formulation, a vinyl or methacrylate class has been shown to be better than the mercapto type. However there is still insufficient published data to guide users in such selection of silanes for peroxide, metallic oxide, amine cures etc.
6. Practical Considerations
1. Users should be aware that selection of the appropriate silica for a formulation is a first prerequisite before thoughts of using a silane coupling agent.
2. For best abrasion resistance as in passenger tyre treads (green tyres), a silica with CTAB of 160-175 m2/ g meets the requirements for good wear resistance, low rolling resistance and low heat build-up.
For carcass compounds where low heat build-up and low rolling resistance are more important than increased wear resistance, a silica with CTAB of 120 m2/ g may be better.
3. Footwear manufacturers commonly use silica with CTAB ranging from 140-175 m2/ g, dictated more by availability and price, rather than on technical basis.
4. It is generally accepted that the higher the CTAB of the silica, the better the wear resistance but the more difficult to process and disperse and the more crepe hardening is to be expected.
5. From theoretical considerations, to avoid variability in service performance, one should stick to one grade of silica or more than one proprietary grade with very close specifications.
6. Poor silica dispersion means agglomerated silica particles which are not reacted with silane and worst still not wetted with the polymer. This will cause besides poor tensile properties, poor wear resistance and high heat build-up.
7. The improved HD silicas like Degussa Ultrasil 7000GR can offer further improvement in wear resistance lower heat build-up and more consistent vulcanisate properties.
8. The effect of silanes on silica in improving wear resistance is quite dramatic. A typical passenger tyre tread with N234 has some 20% better wear resistance than a silica loaded familiar formulation. With silane treated silica, the silica formulation has wear resistance only some 5% lower than the N234 formulation but with much lower rolling resistance and better wet traction.
9. The effect of silanes in silica filled footwear formulations are even more dramatic. In a typical 100 PHR NR, 50 PHR Silica formulations, 1 PHR of MPTES can boost the NBS Abrasion Index by some 40 points. It is no wonder that first users of Si-69 were in solings in the 1980’s before the advent of the green tyres. Little published information are available on the expected improved wet traction of such solings.
10. Other niche applications of silanes such as in improved heat reversion resistant sulphur cures, promotion of adhesion to other substrates, as compatilisers for better polymer-polymer interfacial strength etc will not be dealt with in this Short Note.
11. Selection of silanes for sulphur cured unsaturated polymers centre on the 5 most readily available silanes, TESPT, MPTMS, MPTES, TCPTES an TESPD.
12. Because of the strong pungent odour of MPTMS, it is anticipated that MPTES will be the main mercapto class used in the future.
13. For green tyre compounding where invariably delayed action accelerators are needed, the mercapto class can be ruled out in view of its scorchy behaviour.
14. The preferred silanes in green tyre compounding is still the historical TESPT with its slow curing characteristics and despite its short comings of higher dosage needed & scorch tendency at > 160OC. The improved disulfide grade TESPD is expected to replace TESPT in time – but tyre compounders are known to take a long time to change a formulation. So far TESPT is found to give the lowest tan δ at 60OC compared to other silanes.
15. Because of the known chemistry of silanes, it is understood that silica + silane should be mixed first before the addition of competing chemicals like glycols, amines, zinc oxide and even some antidegradants.
16. In tyre manufacturing, the silanization and hydrophobation reactions need to be taken to near completion by a 2nd Step hot remilling/ mixing, as released alcohol and/ or water can create problems of porosity in extrusion calendaring and even final shaping/ curing of the tyres.
17. In footwear manufacture the above is not so critical as in subsequent moulding of soles, the alcohol and/ or water can easily be bumped off.
18. In footwear manufacturers, the scorchy silane like MPTES is sometimes preferred to give faster curing cycles with less dosage of TMTM needed as compared with TESPT. This is only true if scorch is well under control.
19. It is well accepted that even silane treated silicas filled formulations crepe harden and in remilling of matured storage hardened masterbatches, a lot of frictional heat is generated leading to scorch problems. To avoid this, such stocks should not be matured too long, 4-16 hours should be sufficient, and we recommend warm to hot rolls to start with in final mill mixing.
20. Some reduction in stock viscosity can be achieved with slightly higher dosages of zinc oxide/ stearic acid or PEG4000 which has less tendency to bloom.
21. All silanes are liquid form and prone to hydrolysis. Since compounders loathe to handle unstable liquids some suppliers of silanes offer heat-sealed silane + N330 in EVA bags, wax bound silane in pellet form and even thermoplastic resin bound silanes in pellet form mainly for plastic compounding.
22. We are probably the first to attempt a polymer bound predispersed silane in granular form and the storage stability of our TESPT-50G even after 3 months open ambient storage is still good. (See rheometric comparison in Appendix 1)
23. The different effects of silanes on cure behaviour in a typical transparent soling are illustrated in Appendix1.
Appendix 1
Rheometric Study of Silanes in a
Typical Silica Filled Transparent Outsole Formulation.
Date Tested: 30 May 2000
Rheometer @ 180OC. All silanes added at 2PHR active.
|
|
Control |
HP 669 |
TESPT-50G1 |
A 1289 |
HP 1891 |
SI 264 |
|
Tmin
(N.m) |
1.427 |
1.003 |
1.045 |
1.065 |
1.231 |
1.051 |
|
t2 (min.) |
1.300 |
1.30 |
1.300 |
1.300 |
0.820 |
1.480 |
|
t90 (min.) |
2.380 |
2.740 |
2.680 |
2.680 |
2.780 |
2.560 |
|
Tmax (N.m) |
5.828 |
5.953 |
6.098 |
5.911 |
4.597 |
5.460 |
|
Tmax – Tmin
(N.m) |
4.401 |
4.950 |
5.080 |
4.846 |
3.366 |
4.409 |
Notes: 1. Our Polymer bound TESPT-50G was produced 18 Feb.
2000 i.e. more than 3 months ambient storage.
Comments
1. Both sources of TESPT from Hung Pai HP 669 and Witco A1289 give nearly
similar rheometric data.
2. The scorch safety of the silanes are in the order TCPTES > TESPT
> MPTES. Our own evaluation confirms that use of Mercapto class silanes may
give scorch problems.
3. Higher modulus as expected are given by TESPT as compared to TCPTES
and MPTES.
4. The highest Tmax – Tmin is given by our
TESPT-50G indicating the least loss of active ingredient. For this parameter
our TESPT-50G is 3-5% more efficient than the liquid form.
5. The similarity of 3 months old TESPT-50G with fresh liquid active
TESPT illustrates that its storage stability is good up to at least 3 months
ambient storage.
6. The better scorch safety and yet faster cure time t90 of
TCPTES vs. TESPT is noted.
Table 1. Rheometric Comparison Of 3 Months Old TESPT-50G1 With
Fresh Active TESPT In A Typical Transparent Soling Formulation.
|
Formulation |
Control |
Fresh TESPT |
3 months old TESPT-50G |
|
SMRL2 |
10.00 |
10.00 |
10.00 |
|
NBR3 |
10.00 |
10.00 |
10.00 |
|
BR4 |
80.00 |
80.00 |
80.00 |
|
Silica |
48.00 |
48.00 |
48.00 |
|
ZnO Active |
1.50 |
1.50 |
1.50 |
|
Stearic Acid |
1.00 |
1.00 |
1.00 |
|
Antioxidant BHT |
1.00 |
1.00 |
1.00 |
|
Processing Aid |
2.00 |
2.00 |
2.00 |
|
PEG 40005 |
3.00 |
3.00 |
3.00 |
|
Naph. Oil6 |
14.00 |
14.00 |
14.00 |
|
ISE-75G7 |
3.00 |
3.00 |
3.00 |
|
MBTS-80G8 |
1.00 |
1.00 |
1.00 |
|
TMTM-80G9 |
0.20 |
0.20 |
0.20 |
|
TESPT |
-- |
2 |
-- |
|
TESPT-50G |
-- |
-- |
4 |
Rheometer@180OC
|
|
Control |
Fresh TESPT |
3 months old TESPT-50G |
|
Tmin
(N.m) |
1.427 |
1.003 |
1.045 |
|
t2
(min) |
1.300 |
1.300 |
1.300 |
|
t90
(min) |
2.380 |
2.740 |
2.680 |
|
Tmax (N.m) |
5.828 |
5.953 |
6.098 |
|
Tmax – Tmin (N.m) |
4.401 |
4.950 |
5.080 |
Notes:
1.
Tespt-50G
denotes 50% active TESPT polymer bound in granular form.
2.
Standard
Malaysian Rubber Grade
3.
Butadiene
Acrylonitrile Copolymer
4.
Polybutadiene
rubber
5.
Polyethylene
glycol
6.
Naphthenic
Oil
7.
Sin Rubtech
insoluble sulphur in PBPC form
8.
Sin Rubtech
dibenzothiazole disulfide in PBPC form
9.
Sin Rubtech
tetramethylthiuram monosulfide in PBPC form.